The research goal of our group is to
apply the principles and techniques of quantum optics (e.g. optical trapping, atomic/molecular
coherence, and quantum noise reduction of light) for the detection,
identification, and manipulation of fundamental biological processes at the
level of single cells and single molecules. The current research projects
include:
·
Raman
tweezers
·
Optical
pulling of airborne particles and lifting of large objects by light
·
Monitoring of dynamic biological
process of single cells
·
Identification, characterization and
sorting of living microorganisms
1. Raman tweezers
Raman tweezers is the combination of Nobel-Prize winning
optical tweezers and Raman spectroscopy, which allows capturing and
manipulating a single biological particle including cell, bacterium and virus
and allows acquiring the Raman spectroscopy of the trapped particles. Raman
tweezers can provide biochemical composition of a single living cell without
chemically interfering it and the measured vibrational energy levels
can be used as fingerprints for identification of biological cell.

What are advantages of Raman
tweezers?
Biological cells are the complex mixture of a large number of biomolecules enclosed in cell membrane,
including nucleic acids, proteins, polysaccharides, and lipids. Most cells can live, grow, and reproduce in
liquid growth media, accompanying with continuous changes in biochemical
composition inside the living cells. Identification of biomolecules inside
living cells is very important to understand various cellular processes.
However, the living cell under study may randomly move away from the confocal
excitation volume due to Brownian motion or cell motility and, therefore, the
living cells have to be immobilized either physically or chemically, which will
change the chemical micro-environment of the living cell and may yield unknown
effects on the cell.
Raman tweezers permits the capture of a motile biological
particle in solution without physical contact (without the need of
immobilization), permits optimum excitation and collection of Raman scattering
since the cell is automatically trapped in the focus of the excitation beam,
and permits effective rejection of stray light and fluorescence background.
(Click for a video
of capturing and measuring a moving bacterium)
Standing-wave
Raman tweezers allows stable optical trapping and characterization
of nanostructures (collected in Nature Collection of optical tweezers celebrates the 2018
Nobel Prize in Physics)
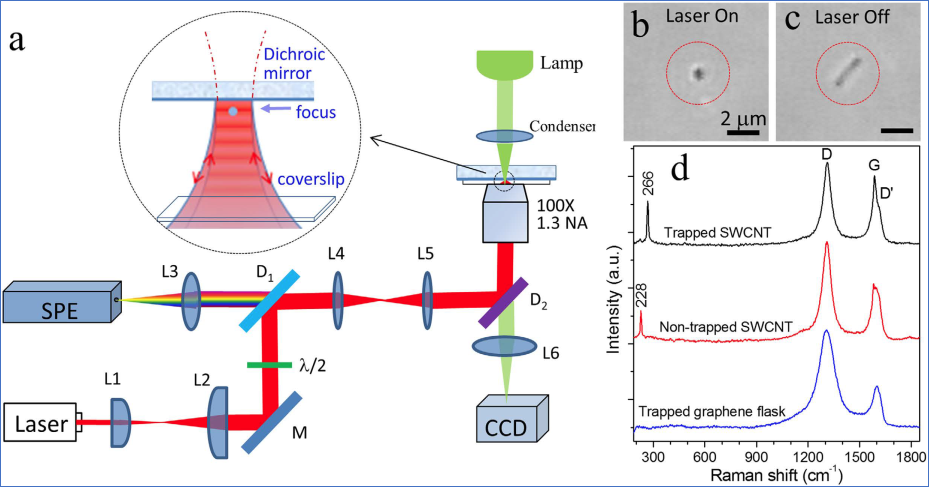
[1]
C.A. Xie, M. A. Dinno, and Y.Q.
Li, “Near-infrared Raman spectroscopy of single optically trapped
biological cells”, Optics Letters, 27, 249-251 (2002). [pdf]
[2] C.A. Xie and Y.Q. Li, “Raman
spectra and optical trapping of highly refractive and nontransparent
particles”, Applied Physics Letters, 81, 951-953 (2002). [pdf]
[3] L.B.
Kong, P.F. Zhang, G.W. Wang, P. Setlow, and Y.Q. Li*,
“Characterization of bacterial spore germination using phase contrast
microscopy, fluorescence microscopy, Raman spectroscopy and optical tweezers”, Nature
Protocols, 6, 625-639 (2011). [pdf]
[4] M.-Y. Wu, D.-X. Ling, L. Ling, W. Li, Y.-Q. Li*, Stable
optical trapping and sensitive characterization of nanostructures using
standing-wave Raman tweezers, Scientific
Reports, 7, 42930 (2017). [pdf]
2. Optical
pulling of airborne particles and lifting of large objects by light
Optical pulling is the
attraction of objects back to the light source by the use of
optically induced “negative forces.” It is commonly expected that when
illuminated by a collimated laser beam, an object will be accelerated along the
light propagation direction by radiation pressure. The
idea of using optical beam to attract objects back to the light source is
counterintuitive and has long been attractive to scientists. We demonstrate
that micron-sized absorbing objects can be optically pulled and manipulated
over a meter-scale distance in air with a collimated laser beam based on
negative photophoretic force.

Optical
pulling of airborne particles over 10 meters. (Click for a video of optical pulling of
airborne particles)
Pulling and lifting macroscopic objects by light
In Maxwell’s theory of electromagnetic waves, light
carries energy and momentum and the exchange of the energy and momentum in
light-matter interaction generates optically-induced forces acting on material
objects. Can a centimeter-sized or larger object that can be viewed by naked
eye be lifted by a light beam? Although optical forces have been widely applied
for the manipulation of microscopic objects, they are usually unnoticeable on macroscopic
objects because the magnitude
of optical forces is generally much weaker than the gravitational force (FG) of the large
objects. Generally, when an object immersed in a gaseous
environment is illuminated by light, two types of optically-induced forces are
generated by the light-matter interaction. One is radiation pressure force (FRP)
arising from direct momentum transfer between the object and the incident
light, which is in pN or nN
range and is not sufficient to lift large objects. The
other is photophoretic or radiometric force (FRM) due to photo-heating effect, in which the photon energy
of the incident light is first converted into the thermal energy of the object
and then asymmetrical momentum transfer between the heated object and the
surrounding gas molecules produces FRM,
which can be several orders of magnitude larger than the radiation force FRP. We directly
observe light-induced attractive forces that allow pulling and lifting
centimeter-sized objects off the ground by a light beam. This large force (~4.4
μN) allows rotating a motor with four-curved
vanes (up to 600 rpm). Optical pulling of macroscopic objects may find
nontrivial applications for solar radiation-powered near-space propulsion
systems.
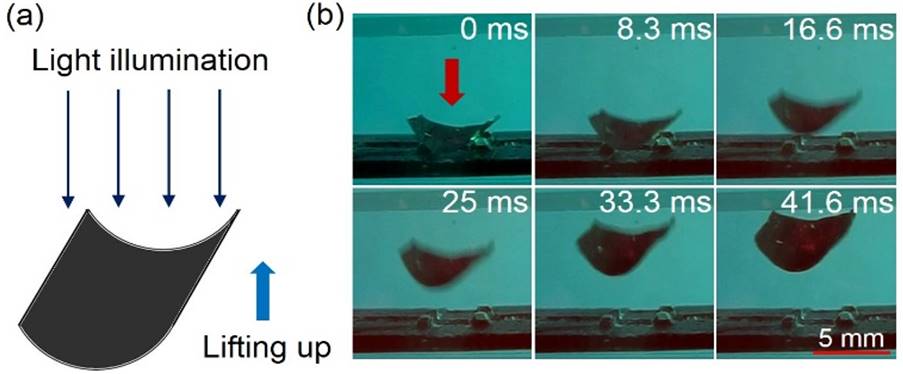
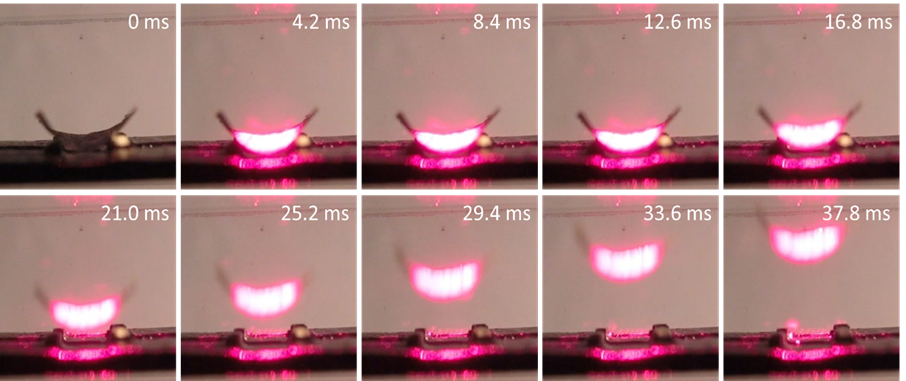
Lifting up of a gold cylindrical vane
(7x7mm) by a 1W of 650 nm laser beam.

(Click
for a video to show optical pulling of a Crookes radiometer with four-curved vanes driven by
light.)
[1] J. Lin, A. G. Hart, and
Y. Li*, "Optical pulling of airborne absorbing
particles and smut spores over a meter-scale distance with negative
photophoretic force," Appl. Phys.
Lett. 106, 171906 (2015).
[2] G. Chen, L. He, M. Wu,
and Y. Li*, "Temporal Dependence of Photophoretic
Force Optically Induced on Absorbing Airborne Particles by a Power-Modulated
Laser," Phys. Rev. Applied 10,
054027 (2018).
[3] L. Ling, Y.Q. Li*, “Measurement of Raman spectra of single airborne absorbing particles trapped by a single laser beam”, Optics Letters. 38(4):416-418 (2013).
[4] J. Lin, and Y. Q. Li,
Optical trapping and rotation of airborne absorbing particles with a single
focused laser beam, Appl. Phys. Lett. 104, 101909 (2014).
3.
Monitor dynamic biological process of single cells and cellular heterogeneity
The ability to monitor biological
dynamics of individual cells and explore cellular heterogeneity is of particular interest to single-cell microbiology. Bulk-scale
measurements report only average values for the population and are not capable
of determining the contributions of individual heterogeneous cells. It is
possible to use micro-Raman tweezers to monitor dynamic biological process and
cellular explore heterogeneity based on measuring the molecular vibration
frequencies from the scattered light. As
an example, we studied on the real-time detection of kinetic germination and
heterogeneity of single Bacillus thuringiensis spores in an aqueous solution by monitoring the calcium
dipicolinate (CaDPA) biomarker with laser
tweezers Raman spectroscopy (LTRS). Germination is the process by which
a dormant spore returns to its vegetative state when exposed to suitable
conditions.
In our experiment, a single B. thuringiensis spore was
optically trapped in a focused laser beam and its Raman spectra were recorded
sequentially in time after the exposure to a nutrient-rich medium, so that the CaDPA amount inside the trapped spore was monitored during the dynamic germination process.
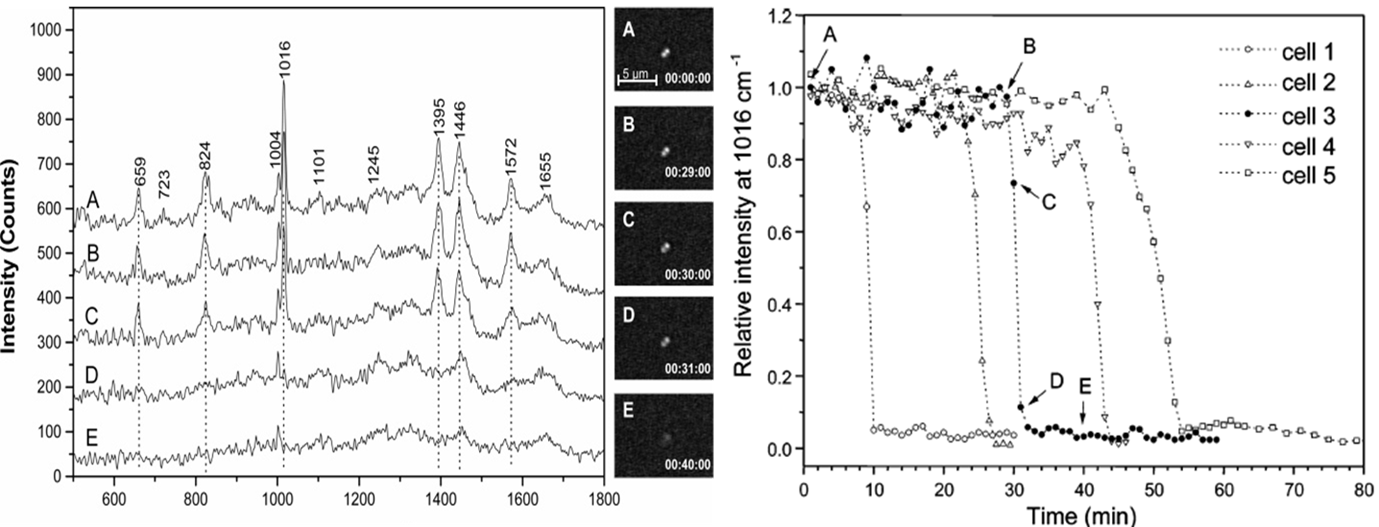
Fig. 1. Time-lapse Raman spectra of a single trapped Bacillus thuringiensis spore after exposure to the TSB growth medium
and the corresponding DIC images. Curve A is for 0 min from the time when the
spore was captured in the optical trap; B for 29 min; C for 30 min; D for
31min; and E for 40 min. Fig.2.
Intensities of the 1016 cm-1 CaDPA band of
five individual Bt
spores as a function of the incubation time.
Live-cell
light microscopy monitors germination, outgrowth, and growth of single bacterial spores
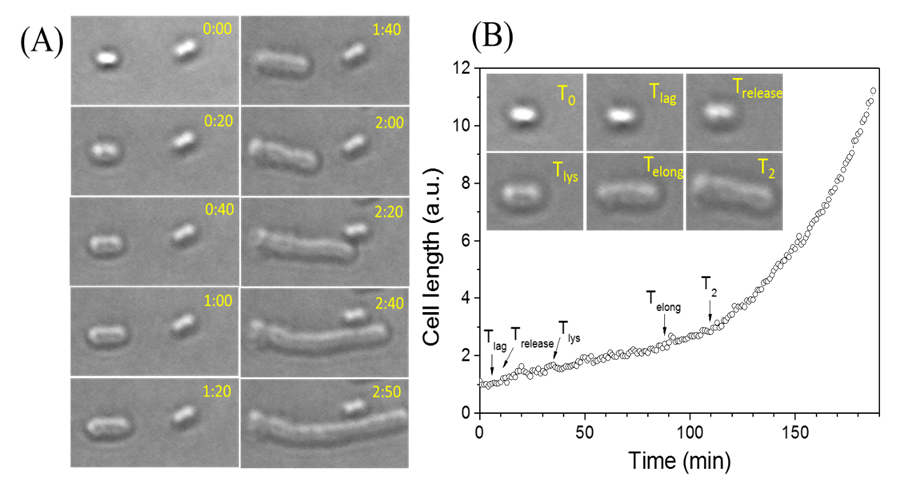
(Click
for a video showing dynamic germination and growing of single individual
bacterial cells)
[1] D. Chen, S.S. Huang, Y.Q. Li. “Real-time detection of kinetic germination
and heterogeneity of single Bacillus
spores by laser tweezers Raman spectroscopy”, Anal. Chem. 78,
2936-6941 (2006). [pdf]
[2] S.W. Wang, B. Setlow, P. Setlow, Y.-Q. Li*,
Uptake and levels of the antibiotic berberine in individual dormant and
germinating Clostridium difficile and Bacillus cereus spores as
measured by laser tweezers Raman spectroscopy. J. Antimicrob.
Chemoth. 71(6):1540-6 (2016). [pdf]
[3] S.W. Wang, J. R. Faeder, P. Setlow, Y.Q. Li*, Memory of germinant stimuli in bacterial spores, mBio, 6(6), e01859-15 (2015). [pdf]
[4] L. He, Z. Chen, S.W. Wang, M.Y. Wu, P. Setlow, Y. -Q. Li*,
Germination, outgrowth, and vegetative growth kinetics of dry heat-treated
individual spores of Bacillus species, Appl. Environ. Microbiol. 84 (7), e02618-17, doi:10.1128/AEM.02618-17
(2018). [pdf]
[5] L.B. Kong, P. Setlow, and
Y.Q. Li*,
“Direct analysis of water content and movement in single dormant bacterial
spores using confocal Raman microspectroscopy and
Raman imaging”, Anal. Chem. 85, 7094−7101 (2013).
[pdf]
4. Identification, characterization and sorting of living microorganisims with Raman tweezers biosensors
Microorganisms
in liquid media can be identified and sorted with Raman tweezers, based on the
intrinsic Raman spectra. Biological and biochemical processes within individual
cells can be monitored in real-time and characterized by using Raman tweezers.
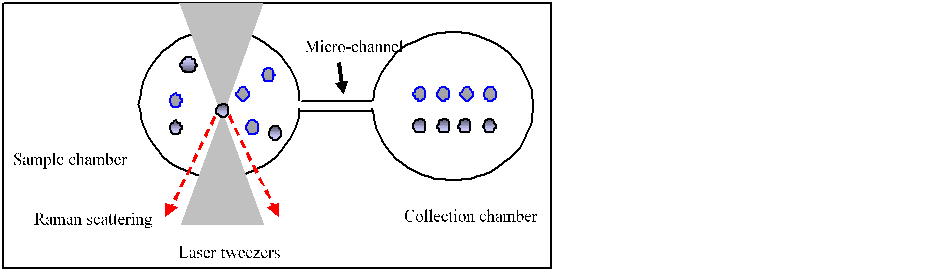
Fig.1
Schematic of LTRS sorting and identification. A particle in the sample chamber
is captured with laser tweezers, identified by Raman spectrum, and then optically
manipulated to a clean collection
chamber.
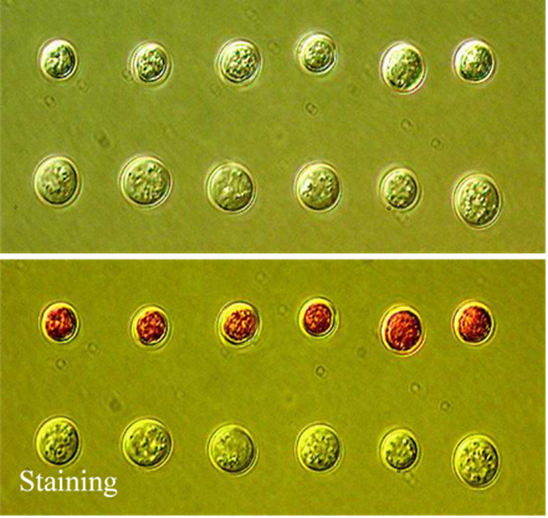
Fig.2 The sorted yeast cells in the collection chamber. The upper row is for dead yeast cells and bottom row is for live yeast cells, identified based on Raman spectra and verified with staining.
[1] C. Xie,
D. Chen, Y.Q. Li, “Raman
sorting and identification of single living micro-organisms with optical
tweezers”, Optics Letters, 30, 1800-1802 (2005). [pdf]
[2] C. Xie,
J. Mace, M.A. Dinno, Y.Q. Li, W. Tang, R.J. Newton, P.J. Gemperline, “Identification of single bacterial cells in aqueous
solution using confocal laser tweezers Raman spectroscopy”, Anal. Chem. 77, 4390-4397 (2005).
[pdf]
[3] M.D.
Mannie, T. McConnell, C.A. Xie, Y.Q. Li, “Activation-dependent phases of T
cells distinguished by use of optical tweezers and near infrared Raman
spectroscopy”, J.
Immunological Methods, 297, 53-60 (2005). [pdf]
[4] K.E. Hamden, B.A. Bryan, P.W.
Ford, C. Xie, Y.Q.
Li, S.M. Akula, “Spectroscopic analysis of
Kaposi's sarcoma-associated herpesvirus infected cells by Raman tweezers”, J .Virol. Methods, 129,145-51
(2005). [pdf]
[5] C.A. Xie,
C. Goodman, M. A. Dinno, and Y.Q. Li,
“Real-time Raman spectroscopy of optically trapped living cells and
organelles”, Optics Express, 12, 6209-6214 (2004).
[6] J. Ojeda,
C.A. Xie, Y.Q.
Li , F. E. Bertrand, J. Wiley, and T.
J. McConnell, “Chromosomal analysis and identification based on optical tweezers
and Raman spectroscopy”, Optics Express,
14, 5385-5393 (2006). [pdf]